Test Code CYS FIB CX Culture, Cystic Fibrosis
Additional Codes
LAB2309
Performing Laboratory
Central Peninsula Hospital
Collection Method
1 swab or sterile container
Container
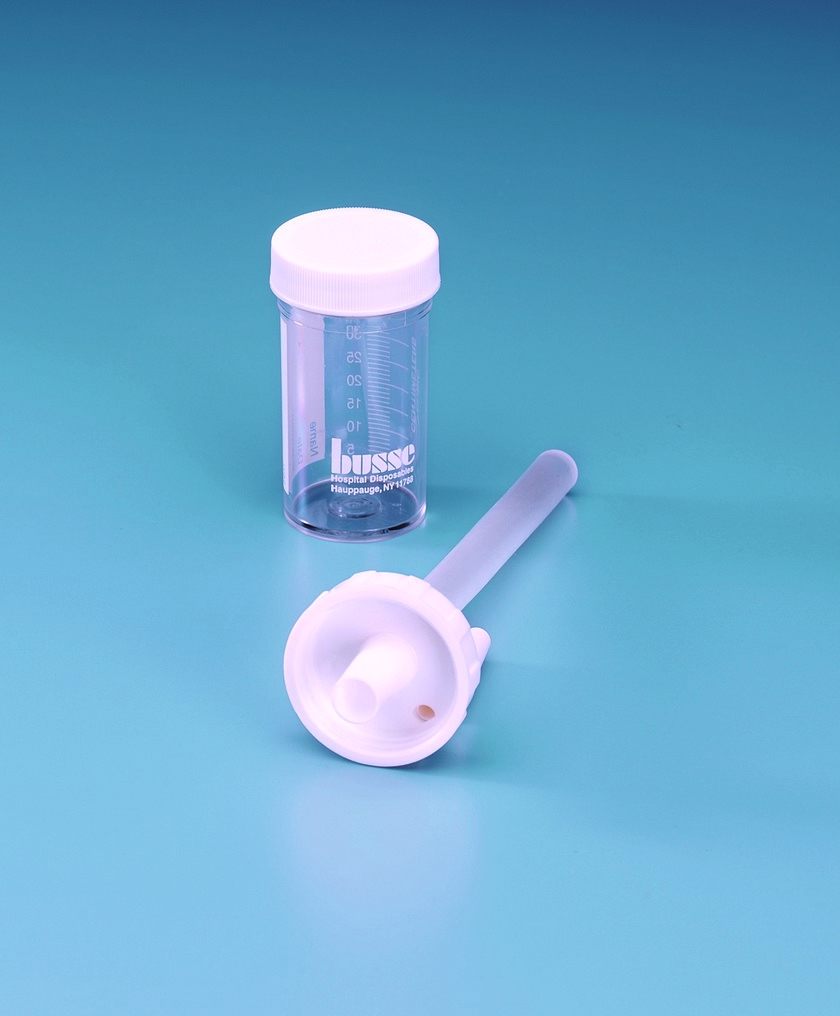

Sterile Container or Eswab
Specimen Type
Preferred
| Specimen Type | Container |
|
Sterile container with leak-proof lid |
Acceptable
| Specimen Type | Container |
|
The following are acceptable if a preferred sample cannot be obtained or is medically contraindicated: • Deep pharyngeal swab • Oropharyngeal (throat) swab |
E-swab; Culture transport swab (Dacon or rayon/nylon swab w/aluminum or plastic shaft in either Liquid Stuart or Liquid Aimes medium.) |
Minimum Volume
1 swab or sterile container
Specimen Stability
Sterile Container
| Ambient | 2 hours |
| Refrigerated | 24 hours |
| Eswab | 48 hours |
| Other swab | 24 hours |
Reference or Target Ranges
No growth
Reportable Units
Growth or No Growth
Critical Value
Not defined for this assay
Reasons for Rejection
- Reject samples collected in non-sterile containers.
- Reject dry swabs without preservative media.
- Reject cotton swabs or swabs that do not meet criteria.
- Reject sputum or swab specimens that have exceeded the stability limits.
- Never reject invasively collected samples, (BAL, aspirates, etc.). Result invasive samples received outside of the stability limits with a disclaimer.
- Do not use sputum rejection criteria for CF samples.
Performance Information
| Days and Time Performed | Daily |
| Expected Turn Around Time | 4 days |
| Stat Availabilty | No |
| Performing Bench | CPH Microbiology |
| Methodology/Method Description | Culture |
CPT Codes
87070
Last review/edit date
2025
